Looking for flashcards for chemistry? Try 21st Night! It allows you to create and share flashcards with anything on them: images, videos, equations, you name it. Plus, there’s a built-in to-do list!
We are better at chemistry now than at any other point in history. In 50 years, we will almost certainly be better at chemistry than we are now.
This statement above is one of the central dogmas of not only chemistry, but of science. In fact, it’s so central that it probably just seems factual. Of course we’re better now, right? We have the benefit of history!
It’s easy to forget or not realize how controversial this statement would have been even 400 years ago, though. Scholars then were raised in a culture that worshipped the achievements of their intellectual ancestors. Claiming that you knew more than Aristotle about science was equivalent to claiming you knew more than Paul about Christianity. I mean this literally. One of the reasons Galileo was famously imprisoned wasn’t simply for disagreeing with the church, but disagreeing with the words of the “Philosopher”, Aristotle. Aristotle, if you’re unfamiliar, lived approximately 2000 years before Galileo.
I am glad that we no longer live in that era. That was a worse time for science and a worse time for humanity. On a moral level, I abhor the idea that intellectual opinions can be censured by imprisonment or death. On an intellectual level, I think an obsession with the achievements of the past necessarily inhibits recognizing advancements in the present, and leads to stagnation.
But the pendulum has swung too far the other way. Specifically, I think the “obviousness” of the inevitability of scientific progress, even though it’s well-founded, tends to cloak that the progress of the associated scientific culture is by no means inevitable. For instance, there have been some recent great essays arguing that our research culture has regressed, at least judging by the number of people involved versus our rate of scientific advancements.
What I’d like to argue in this essay is that, similarly, the obviousness of progress in chemistry has masked the regression of the associated culture of teaching chemistry, especially general chemistry.
It’s regressed in the sense that students today come out of general chemistry with a mistaken view of what chemistry is, and lacking in practical chemistry skills. In the past, students have come out of general chemistry with a better idea of the principles of chemistry, and with greater practical skills.
A big reason as to why today’s students are mistaken in their view of chemistry can be seen in the texts we use. In modern chemistry textbooks, very few portions are justified by empirical evidence. They are justified by principles and deduction, and occasionally illustrated by real-world examples. But, it is incredibly rare to see citations from the original laboratory work that proved the theories.
Meanwhile, the practical side of chemistry (as well as the emphasis of the importance of it) gets let down by the hands-on portion of chemistry, laboratory work. Laboratory work is meant to be training in empiricism. However, this empiricism is only in theory. The laboratory work of introductory chemistry is closer to a cookbook than an experiment. Students are given incredibly precise instructions and told to find a certain result. If they fail to find the result, they are made to redo the “experiment”.
The textbook and laboratory work combined leave a general chemistry student today with two takeaway impressions:
1. Chemistry is a monumental edifice of theory and deduction, disconnected to empirical evidence.
2. When empirical evidence does conflict with chemical theory, the evidence must change.
These takeaway impressions are regressive. These would be the explicit lessons of Aristotelian chemistry, which distrusted experimentation except as illustration. When the ancient Greeks discussed chemistry in terms of the four elements of fire, air, water, and earth, they would illustrate the similarity of fire and air by discussing candles. However, it was literally anathema to the Greeks to disprove the theory of the elements by experiment.
So, if chemistry teaching has regressed, then we need to return it to its former glory by progressing it again, right?
Before our current shoddy state, chemistry teaching was much better at impressing upon students the empirical basis of chemistry. Even more importantly, they impressed upon students the importance of doing chemistry, rather than simply learning it and keeping your lecture notes in a notebook on a shelf. That is the standard of teaching I’d like to go back to, which I’ll explore more later on in this essay.
Before I get there, though, I want to explore how we’ve gotten to where we are, starting from the beginning of chemistry teaching. It would behoove us to be mindful of our history, especially as we’ve already started to repeat it.
But before even that, I wanted to get a few things out of the way.
First of all, my qualifications: I took chemistry in high school and college, both general and geochemistry (I was a geosciences major). I found general chemistry very boring, geochemistry less so. Since college, I’ve worked as a philosophy teacher and own a test-prep business. While I’m not the most qualified to discuss chemistry, I am very qualified to discuss education, and would like to frame this essay as such.
Second, I wanted to define chemistry as the science of chemicals, and the teaching of chemistry as teaching what to do with chemicals. The early years of chemistry had a lot of overlap with philosophy, and more recently chemistry has had a lot of overlap with physics. I’d rather not discuss those right now, so my historical tour is limited to chemicals only.
Finally, it can be a bit tough to get information on what the live teaching of chemistry was like (there weren’t reporters), so I have to rely a lot on textbooks and surviving notes.
With that in mind, I’d like to go back to the first chemistry textbook, and the first hints of tension between students learning chemistry as a theory vs. students learning chemistry as an empirical science. It was when the teaching of chemistry was still in progress…
***
The first chemistry textbook was, well, a weird one. 500 years ago, chemistry wasn’t a subject. Really, there weren’t many distinct subjects at all, as most things science-like were subsumed under broad category “natural philosophy”.
This wasn’t just a nomenclature thing. Because so many subjects were under one umbrella, the techniques and even boundaries of chemistry were shared with a bunch of other subjects. So, we are forced to rely on the definition of chemistry mentioned previously, and define the first chemistry textbook as “the first book that systematically discussed and taught how to use chemicals”.
Using that definition, we find ourselves in the mid 1500s in Bavaria with Georgius Agricola’s De re metallica.
For those of you who are familiar with Latin, you might already see the problem. The title of the book translates to “On the Nature of Metals”, and it was mostly about the operation of mines. This included political instruction, like “Don’t start mines in authoritarian states”, and practical engineering details, like how to make sure your mineshafts don’t collapse.
Crucially for chemistry, though, it also included how to get your ores out of your minerals. Whenever a mineral is mined, it doesn’t come in a pure form. It almost always comes in the form of a weird rock.
So, how do you get usable minerals out of a weird rock? With chemistry! Or, as Agricola would put it, the manufacture and use of “juices”, like alum (a salt), “vitriol” (sulfuric acid), and sulfur.
Agricola, in his instructional work on mining, therefore also taught chemistry. He didn’t teach chemistry in any traditional way. He was really just a reporter. He reported what the miners did (and included diagrams), without explanation or theory. He didn’t even use units. He would just say things like “leave vitriolous stones out in summer and winter” until they “get soft”.

But it is precisely this approach to teaching chemistry that made Agricola a seminal figure in the teaching of chemistry. Before Agricola, chemistry (or, to be precise, the parts of natural philosophy that dealt with chemicals) was entirely theoretical. Evidence had zero impact on theory, and theory was deductive and based on intuition and first principles.
That’s why Aristotle’s chemistry was based on the four elements of air, fire, water, and earth. It made sense to him, and then he could create all sorts of fun diagrams. Then students were taught to memorize them, and replicate them using deduction. By the time we got to chemical “equations” like dry + hot = fire, we’ve created an entire theory which doesn’t help us understand the actual reactions at all.
Agricola just ignored all of it, despite the fact that it was considered the foremost science of his time and something he almost certainly learned. There is nothing in Aristotle’s chemistry that could explain why the miners’ processes to create sulfuric acid worked. Any attempt to use Aristotle’s theory to do so would just be misleading.
So, if we think about what chemistry students would take away from this work, we can say their takeaway would be something like “In order to make use of chemicals, you have to follow a process that is known to work.”
It’s not a great foundation to science, but it’s a start. It’s a place to build from, and, more importantly, puts the use of chemicals at the forefront of chemistry. But it’s a long ways away from good chemistry teaching.
On a side note, I read De re metallica in an English translation, translated by none other than Herbert Hoover (former President of the US and mining engineer) and his wife. Hoover was an absolutely fascinating and incredibly impressive person, and it is really a pity that he screwed up handling the Great Depression so bad as President.
***
The next big innovation in chemistry teaching came from an unlikely source: yet more theorists. This time, not the Aristotelians, but the alchemists. Yes, those alchemists, of eternal life and transmutation into gold renown.
Alchemy had been around for a while, but, by the 1500s, it was the rage among intellectuals. Even Newton was obsessed with it. Now, the theoretical foundations of alchemy were total nonsense, much like the theoretical foundations of Aristotelian science. For example, Paracelsus, one of the founding fathers of Renaissance alchemy, believed that all diseases came from sulfur, salt, and mercury, which he “proved” by burning wood. It’s hard to imagine being more wrong, no?
But the alchemists, despite their foundations being flawed, did have a distinct advantage over the Aristotelians in both their chemistry and chemistry teaching. The alchemists were meticulous experimentalists, precise and excellent at record-keeping. That meticulousness and desire to repeat experiments is exactly why they were responsible for the next big innovation in chemistry teaching. They just needed to be bold enough to throw out theory entirely.
That is precisely what Jean Beguin, a French alchemist, did. In 1610, he published a chemistry textbook that represented the best of the alchemists, called the Tyrocinium Chymicum, or “tournament of chemicals”. It was a guide to manufacturing pharmaceuticals (there was a lot of overlap between medicine and alchemy, as the Paracelsus example shows), and it was a big step forwards in that it was the first chemistry textbook to actually use units (hurray!).
Well, Beguin didn’t exactly publish his textbook. He actually shared it privately with his students. Then it was pirated, became incredibly popular, and Beguin improved and republished it under the idea that, if it was going to be popular, it might as well be good. This does raise the alternative topic of the connection between piracy and improved scientific education, but that can be tabled for now.
Tyrocinium Chymicum represented the best of the alchemists because it is bold. It takes the experimentation of the alchemists and throws out the rest.
At this time, this was not an easy thing to do. Beguin emphasizes in his preface that he has nothing but respect for alchemy, and for Galen, Hippocrates, and the whole Greek tradition of science. He also emphasizes that, because it’s obvious he respects the whole tradition, he requests that other alchemists stop getting so upset with him. The subtext, of course, is that he would really appreciate especially if they wouldn’t somehow get the church involved, as he didn’t wish to be Galileo-d.
After emphasizing his respect for alchemy and the Greek tradition in his preface, Beguin proceeds to not discuss it for the rest of the book. The entire rest of the book is just incredibly detailed cataloguing of how to produce various pharmaceuticals. And, unlike Agricola, Beguin’s alchemical background let him be very, very detailed. In Tyrocinium, there are never any descriptions like “put rocks in a ditch and wait”. There are weights, instruments, and a lot of precise cataloguing of inputs and outputs.

The fact that Beguin was willing to throw out theory meant that this was actually a very effective way to teach how to use chemicals. For example, Beguin had no idea why soaking oak and filtering it produced a liquid that could dissolve pearls. That would require him knowing what tannic acid was, and then understanding how it related to pearls (calcium carbonate). If he tried to understand it using alchemical theory, he’d get confused.
But, he didn’t understand it, and he didn’t really care why he didn’t. He just described it. Exhaustively. In minute detail. Making sure not to forget literally any step even if he had no idea if it was important.
He does, unfortunately, prescribe literally everything he creates for some ailment or another. So, for instance, he prescribes putting ammonium hydrosulfide directly into open wounds (pg 28), which, if you’re unfamiliar, is the active ingredient in stink bombs. He was a better chemist than physician.
Tyrocinium Chymicum is not a fun read, even when you compare it to other alchemy books. But it’s an incredibly important text in chemistry. It is a book by someone who experimented excessively with chemicals, laboriously detailed everything he found, and then instructed others so they could do the same, even under fear of censure/imprisonment/death. It also contains the glimpse of chemical equations in his excessive cataloguing, the first big step in useful chemical theory.
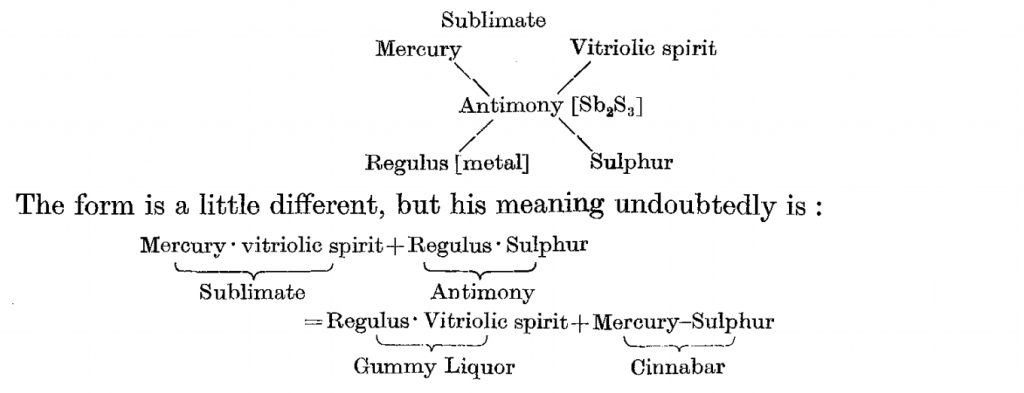
So, if we think back to the idea of takeaways by chemistry students, we might get something from Tyrocinium like, “Chemistry is about following steps meticulously, using the proper instruments, and exhaustively recording inputs and outputs”. Funnily enough, this isn’t so different from our chemistry labs today.
So, we’re at the 1600s, and we’ve already reached the state of laboratory work today in general chemistry. If that’s how far we’ve regressed, then it is worthwhile to see what we’ve given up.
***
Human beings are natural categorizers, and we’re good at it. It’s easy to mistake that with being good at science. Aristotle certainly did, categorizing elements as “hot” or “vaporous”, fooling himself and much of the Western world (up to the mid 1600s!) into not noticing that the categories said more about man’s perception than the elements themselves.
By the mid 1700s, categorization had come roaring back into chemistry teaching. This time, at least, it was backed up by the rigorous experimentation exemplified by Beguin. Unfortunately, it still came with the baggage of theory, although it fortunately no longer got in the way of experimentation quite so much. For instance, it was much more unlikely that you’d get thrown in jail for your experimental findings disagreeing with theory.
The year was 1766, and our hero Joseph Black, a brilliant scholar and professor in the midst of the Scottish Enlightenment. The Scottish Enlightenment, if you’re unfamiliar, was one of those strange moments in time when all of the world’s most influential thinkers were, for some reason, in one place. In this case, it was Edinburgh.
While we don’t have any textbooks from that time, we do have copies of lecture notes that people took in Black’s classes. Black was very popular as a lecturer, and people would actually attend just for fun. A big part of this was his innovative use of flashy experiments to illustrate his points.
This is more important than it seems. Black’s lectures, by focusing around experiments, made it clear to the students that chemistry, in the end, was about describing reality. The lectures helped make sense of what the demonstrations showed. Students imbibed the importance of empirical evidence.
Another big innovation of Black’s time (if not Black himself) was, as previously mentioned, useful categorization. Agricola and Beguin threw out the categorization of Aristotle and Paracelsus (despite Beguin’s protests to the contrary) because they simply did not correspond to reality. It’s one thing to say burning wood shows the unification of sulfur, mercury, and salt, but if you’re actually an experimentalist, you know that’s dumb. However, once they threw it out, they didn’t have anything to replace it with.
Black and his brethren, along with further developing the rudimentary chemical equations originated by Beguin, developed a new type of categorization: affinity tables. They knew certain elements ended up bonded with other elements. They didn’t really know why, or have a good way of predicting future bonds besides noticing obvious similarities between elements.
So, that’s what they did. They grouped elements by similarities, and created tables of which elements bonded with which other elements. As a teaching tool, this was handy, in a sort of similar way to memorizing the periodic table of elements.

As mentioned, though, this came with the burden of theory. Students learned that bonding was due to an invisible force called “affinity” (hence, affinity table). One can only assume that students who learned this and scholars who studied affinity theoretically massively wasted their time. Oh, well.
So, here’s what the students of Black would take away from his classes:
1) Chemistry is an interesting, useful subject founded upon experimentation.
2) Theory is a useful tool for a chemist when it’s grounded in experimental results.
This is getting closer to what a chemistry student should believe, and better than the impressions a student would get from general chemistry today. However, what we’re still missing is a sense of all of the uses of theory (although Black himself wasn’t aware of them), as well as the connection of theory not just to usefulness but to reality.
***
After the 1700s, chemistry was set on a clear trajectory. An openness to scientific inquiry and state sponsorship meant that the inevitability of scientific progress was basically set at this point. Meanwhile, this steady march of improved understanding led to an appreciation for scientific knowledge in general, and a great atmosphere for scientific learning.
Here’s where I say something controversial.
The atmosphere was so great, in fact, that the teaching of general chemistry actually reached its peak in the mid 1800s. The mid 1800s marks our point of regression.
Gasp!
Let me explain.
The mid 1800s were a unique point in chemistry. The usefulness of chemistry had exploded. Chemical industries were in full swing. The universities incorporated chemical labs. In Europe, the state sponsored research and development, while in America, the state sponsored intellectual piracy of European research and development.
Chemical theory, meanwhile, had actually become really useful! In Germany, Augustus Kekulé discovered and popularized the use of diagrams for organic chemistry. Simultaneously, the careful experimentation of laboratory scientists meant that categorizing chemicals could actually be done in a systematic way (like by weight of each element in a given chemical). Eventually, by the mid-late 1800s (1869, to be precise), the ultimate chemical categorization tool arrived: the periodic table.
Now, what’s interesting is that a lot of what we consider “fundamental” to chemistry was still not on the table (pun intended). Gilbert Lewis introduced the idea of electron pairs only in 1916. In fact, the electron wasn’t even discovered until 1897, and the nucleus in 1909. We’re still 50 years out from all of that.
So what did that mean for chemistry students? Well, it meant that their chemistry instruction was incredibly practical. Theory wasn’t developed enough to be learned solely on its own, so it was taught to supplement the laboratory work. Germany especially was a paragon of practical chemical learning with its massive research laboratories. The “Giessen method” of teaching was developed during this time by Justus von Liebig, one of the foremost chemists of his day. It involved massive amounts of laboratory work, hands-on training in what chemists actually do.
Crucially, this wasn’t just cookbook laboratory work, like we do today. These were legitimate experiments, closely tied to the active research von Liebig was carrying out at the time. The assignments would give instructions as to the goal, but only vague instructions on how to carry it out.
The final exam for the end of a student’s chemistry education was the so-called 100 bottle challenge, where a student had to analyze 100 bottles of unknown compounds for their constituents. The reward once you’re done? Well, join von Liebig’s lab as an assistant, of course!
As you can tell, every piece of a student’s chemistry education under the Giessen method was to prepare you to go out in the world and do chemistry. Students were in the lab from the beginning, learning theory and performing experiments that actually helped them do chemistry.
That’s why I say this is the best time for chemistry teaching. Here’s what a chemistry student’s takeaways would be:
1) Practical methods on how to do chemistry as taught by someone who was at the forefront of research chemistry.
2) Specific theories, as well as how to apply them, why they’re useful, and the empirical evidence supporting them
In this era and place, general chemistry students were taught to the greatest extent possible to be chemists. That, to my mind, is the greatest possible way to teach chemistry: not as something to learn, but as something to do.
***
If the Giessen method was indeed the peak of chemistry teaching, then it’s all downhill from there (as peaks tend to operate). That’s unfortunate, because next up on our historical tour of chemistry teaching is Linus Pauling.
I really shouldn’t throw Linus Pauling under the bus. The man was incredibly brilliant, and an absolute font of ideas, some Nobel Prize winning and only a few that can be described as stupid (e.g. using Vitamin C to cure AIDS). And his approach to teaching chemistry, especially general chemistry, was pretty clever.
Pauling, somewhat similarly to Joseph Black, saw introductory chemistry as serving two functions: introducing chemistry and making people interested in it. So, that’s how he set up his teaching and his textbook.

When it came to introducing chemistry, Pauling took the approach of building up chemistry logically, like the textbooks of today, by starting with atoms and working his way up to chemical bonding. However, unlike the textbooks of today, he still included the empirical evidence that supported the fundamental assertions.
For instance, when introducing the periodic table, Pauling puts it in, explains it, and then briefly talks about the experimental evidence that confirmed it. He doesn’t simply cite facts referenced in the book (like similarities of alkali metals, as a modern chemistry textbook would do), but references the actual experiments done and includes their results.
It’s when dealing with what makes people interested in chemistry, though, that Pauling’s love for science really shines through and makes an impact on his students. He obviously loves collecting interesting and illustrative reactions and properties. It’s clear that he isn’t just familiar with chemistry as an academic subject, either, as he weaves in the use of chemicals in engineering as well.

Where does his teaching fall short, though? Well, exactly where Justus von Liebig’s work shined: as an example of how to do chemistry. The practice problems in the book are abysmal, rote, and seem to mostly exist to fill space. They are problems that in no way are natural ones to come up, or to trigger deeper thought from the students.
So, then, what would the takeaways be from Linus Pauling’s course as a student?
1) Chemistry is logical, interesting, and based on empirical evidence
2) The empirical evidence that chemistry is based on is beyond the ability of introductory chem students to repeat
In 100 years, this was the shift. Chemistry, as something that one did, was relegated to more advanced courses. You would no longer be able to do chemistry by taking an intro course alone.
The regress, then, would come in disregarding the empirical evidence entirely, and transforming chemistry entirely into something that one learned, rather than did.
***
This brings us to the present day, the year of our lord, 2020.
As mentioned, I took high school chemistry and 2 semesters of college chemistry. I don’t remember what textbook I used for my course, but it was much like this free online textbook, Chemistry 2e, for both my high school and college classes.
With this textbook, we’ve moved completely away from chemistry as something to do to chemistry as something to learn. The book is illustrated with plenty of examples, sure, but not of actual experiments that any real scientist did to prove or explore concepts. These are examples that literally only exist within the bounds of a chemistry textbook, the sort that use color changes with easy chemical formulae.

It’s sterile chemistry. It’s the equivalent of teaching kids scales before they learn to play a song, or insisting that kids learn grammar before teaching them how to write. It’s chemistry as rote and rules, with no joy to exploration.
What’s frustrating about this is that it isn’t wrong, and that’s what its proponents hide behind. This is a way to teach students chemistry so that they learn the right things. They learn an introduction to everything in order, and learn the right facts to make sense of it all.
But, at the end of it, there’s nothing to take away. There’s certainly nothing that a chemistry student can do with this knowledge. It’s as useless as the dates he learned in history class or the declensions he learned in Latin. It’s another thing that will simply molder away in a notebook until it’s thrown out.
It doesn’t have to be this way. In fact, it wasn’t supposed to be this way. While researching for this essay, I stumbled across the CHEM project, which was a project in the 1960s to modernize the teaching of high school chemistry. While it’s not perfect, its emphasis on teaching chemistry that is grounded in the sort of experiments that high schoolers can do is an improvement.
Unfortunately, from what I can tell, it never got much traction. A follow up survey in the 70s found only 20% of high schools using the curriculum, and I’m not sure if any do now.
So, the takeaways, then, for a modern college chemistry student is this:
1) Chemistry is logical.
2) The empirical evidence for chemistry is unimportant.
3) The applications of chemistry are for changing the colors of solutions, and for describing things that are already kind of obvious.
4) Chemistry experiments are about repeating a process until you get to a specified endpoint
Students walk out of modern general chemistry classes finding chemistry neither interesting nor understanding its use. These classes are a process repeated over and over again, in college and high school classrooms around the world, with no clear goal or even metrics. They are closer to rituals than anything else.
As I’ve hopefully shown, this is a relatively recent phenomenon. Before the past 100 years or so, theory wasn’t developed enough to dominate any class, never mind general chemistry. As a result, classes could be far more practical, not only engaging the student but teaching them chemistry as something useful.
If nothing else, I hope we can think about going back to that time. Chemistry teaching should be held to as high of standards as chemistry itself, and not left simply to wither.
What a wonderful essay. I’ve often thought that the way we teach science in school is so at odds with how science is actually done that it’s amazing any scientific progress has been made at all.
The point you make about acid rain was the key inflection point for me. Science is, if nothing else, a labour of love. By instructing students in this way, teachers are inflicting a learned helplessness on their classes that says “I don’t know how to do this yet” instead of teaching them to think “that’s interesting, I wonder how it works?” You earned yourself a reader, I look forward to future pieces.
Good essay, thanks. As an aside, the apparently useless work of the alchemists may in fact not be completely useless. You said in the essay that *”the categories said more about man’s perception than the elements themselves.”*. Jung also observed this and he thought the alchemical writings were useful insight into peoples imagination, which he then mined for information. So perhaps it was bad chemistry but useful for psychology.
Thank you — you’ve helped me understand why I always found chemistry boring in high school (and, because of that, avoided it in college)!
If I, too, can step in to defend the alchemists (and how did these comments become a hotbed of alchemy apologetics?!), Larry Gonick (in *The Cartoon Guide to Chemistry*) suggests that the alchemists developed the nuanced practical skills (of e.g. measuring, heating, mixing…) that provided the basis for modern chemistry.
(A fun history-of-chemistry side note: Gonick also suggests that what held the alchemists from a major breakthrough was a lack of rubber stoppers! When they could stop solutions from invisibly evaporating, they could figure out that mass was conserved through chemical reactions.)
I think it all comes down to the instructor. I took Organic the t time with a semi-famous professor who didn t want to be there and just sat next to an overhead projector and wrote down formulas for us to memorize. Boring! I squeaked by with a C. When I had to take it again for grad school because I failed the organic part of the entrance exams (imagine that) I had a completely different experience. The instructor obviously loved to teach and she taught us the principals as to why A+B=C rather than just memorizing. I loved that class and got an A. Organic and inorganic chemistry are almost two separate subjects. I also took organic freshman year in college with a professor who I think probably ended up in the running for a Nobel Prize. I m not sure he was particularly bad but it was a large lecture class, I hated organic and it was one of the first subjects I ever didn t really grok at some level, and actually somewhat changed majors because of it.